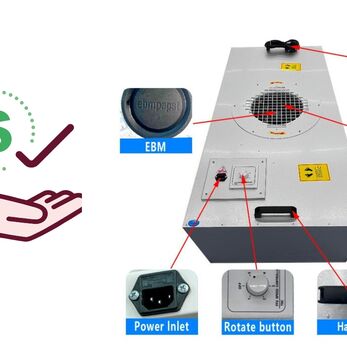
FFU – Fan Filter Unit is an air filter with integrated fan, helping to maintain a clean environment that meets GMP standards. This article introduces the GMP-standard FFU – Fan Filter Unit supplier in Vietnam, meeting the needs of clean room installation in pharmaceuticals, food, cosmetics and electronics.
- 1. Introduction to FFU and GMP Requirements
- 2. GMP Standards Applied to FFU
- 3. Advantages of Using GMP-Compliant FFUs
- 4. Why Choose VCR as Your FFU Supplier in Vietnam
- 5. Comparison Between Standard FFU and GMP-Compliant FFU
- 6. Frequently Asked Questions about GMP-Compliant FFUs
- 7. Contact for Consultation & GMP-Grade FFU Quotation
1. Introduction to FFU and GMP Requirements
What is FFU (Fan Filter Unit)?
FFU - Fan Filter Unit is an air filtration device with an integrated fan. It draws in air from above, filters it through a HEPA/ULPA membrane, and blows it downward in a laminar flow. This helps maintain cleanroom air quality by removing fine dust, microorganisms, and airborne particles.
Compared to traditional air filtration systems, FFU is compact, easy to install in modular form, and energy-efficient, making it suitable for both large-scale cleanrooms and localized clean booths.
The Role of FFU in GMP-Compliant Cleanrooms
In pharmaceutical, food, and cosmetic factories, GMP (Good Manufacturing Practice) requires strict control of air quality. FFU plays a critical role by:
- Ensuring a stable cleanliness level in compliance with ISO 14644 and GMP.
- Preventing cross-contamination between sensitive production areas.
- Supporting microbiological control in weighing, filling, and packaging zones.
- Optimizing operations with easy replacement, maintenance, and flexible installation.
In other words, FFU is the “heart” of a cleanroom air filtration system, helping businesses maintain product quality and comply with international GMP regulations.

Industries that Require FFU
FFU is widely applied in industries with strict environmental control:
- Pharmaceuticals: ensures sterility in drug production, weighing rooms, filling, and packaging.
- Food: prevents cross-contamination and microbial growth in processing lines, especially for perishable or fermented products.
- Electronics & Semiconductors: maintains ISO 5-7 cleanliness to protect sensitive components from dust and static.
- Cosmetics: complies with ISO 22716 and GMP, protecting filling and packaging processes in a clean environment.
With such importance, FFU is indispensable in the construction and operation of GMP-certified cleanrooms.
2. GMP Standards Applied to FFU
Requirements for Dust and Microbial Control
In cleanroom systems, GMP standards set strict requirements for air cleanliness. FFUs must:
- Control airborne particles: remove fine dust ≥0.3 µm, maintaining particle counts according to ISO 14644 classifications.
- Reduce microbial contamination: minimize bacteria and mold in production spaces.
- Maintain pressure and laminar flow: prevent cross-contamination between different cleanroom zones.
This is particularly crucial in pharmaceutical and food industries, where even a single particle or microorganism can compromise product quality.
Relevant Certifications and Standards
A GMP-compliant FFU often needs to meet multiple international standards:
- EU GMP (European Union GMP): requirements for cleanroom design and operation in pharmaceuticals.
- WHO GMP: World Health Organization guidance on air quality control in drug manufacturing.
- ISO 14644: international standard for cleanroom classification (ISO Class 1-9).
- ISO 22716 (Cosmetic GMP): requires a clean environment for cosmetic filling and packaging.
Compliance with these standards ensures that companies not only meet audit requirements but also guarantee safe, high-quality products in global markets.

Technical Features of GMP-Compliant FFUs
A GMP-grade FFU typically offers:
- HEPA/ULPA Filtration
- HEPA H14 (≥99.995% efficiency for particles ≥0.3 µm).
- ULPA U15-U17 (≥99.9995% efficiency for particles ≥0.12 µm).
- Stable Airflow
- Air velocity of 0.3 - 0.45 m/s at the outlet.
- Maintains positive pressure in GMP cleanrooms.
- Low Noise Level
- Below 55 dB, suitable for continuous production.
- Durable Materials
- Powder-coated steel or stainless steel 304, easy to clean and corrosion-resistant.
- Modular Design
- Flexible installation: ceiling-mounted, clean booths, or production lines.
These features ensure that FFUs not only comply with GMP but also enhance equipment longevity and reduce operating costs.
See more: HEPA Filter Leak Testing in FFUs – Fan Filter Unit
3. Advantages of Using GMP-Compliant FFUs
High Filtration Efficiency and Stable Cleanliness
- Reliable cleanliness levels: HEPA/ULPA filtration eliminates up to 99.995% of ultra-fine particles, achieving ISO 5-7 cleanroom standards.
- Prevents cross-contamination: laminar airflow ensures contaminants do not recirculate, crucial for filling and packaging areas.
- Stable long-term performance: FFUs support compliance with stringent inspection and validation requirements.
Flexible Installation Options
- Ceiling-mounted systems: integrated into cleanroom ceilings, saving space and maintaining a uniform surface.
- Modular design: allows easy expansion or replacement when production needs change.
- Clean booth applications: ideal for localized clean environments such as weighing and compounding areas.
This flexibility makes FFUs suitable for both new facilities and retrofit projects.
Optimized Operating and Maintenance Costs
- Energy efficiency: many GMP FFUs use EC (Electronically Commutated) motors, reducing energy consumption.
- Easy maintenance: HEPA/ULPA filters can be replaced without dismantling the entire system.
- Long-term cost savings: durable construction, fewer breakdowns, and reduced repair costs.
Thanks to these advantages, GMP-grade FFUs not only meet technical requirements but also deliver sustainable economic benefits for manufacturers.
4. Why Choose VCR as Your FFU Supplier in Vietnam
Over 10 Years of Experience in Cleanroom Solutions
VCR is one of the pioneering companies in Vietnam specializing in cleanroom equipment and solutions. With more than a decade of experience, VCR has participated in hundreds of GMP projects for pharmaceutical, food, cosmetic, and electronics factories, consistently meeting the strictest validation and audit requirements.
A Trusted Partner Across Multiple Industries
VCR has become a reliable partner for many leading enterprises in different sectors:
- Pharmaceuticals: supplying FFUs for facilities certified under EU-GMP and WHO-GMP.
- Food: providing microbial control solutions for processing lines.
- Electronics & Semiconductors: FFUs certified to ISO 5-7, protecting sensitive components and microchips.
- Cosmetics: cleanrooms for filling and packaging in compliance with ISO 22716.
This cross-industry presence demonstrates VCR’s capabilities and reputation in the Vietnamese market.
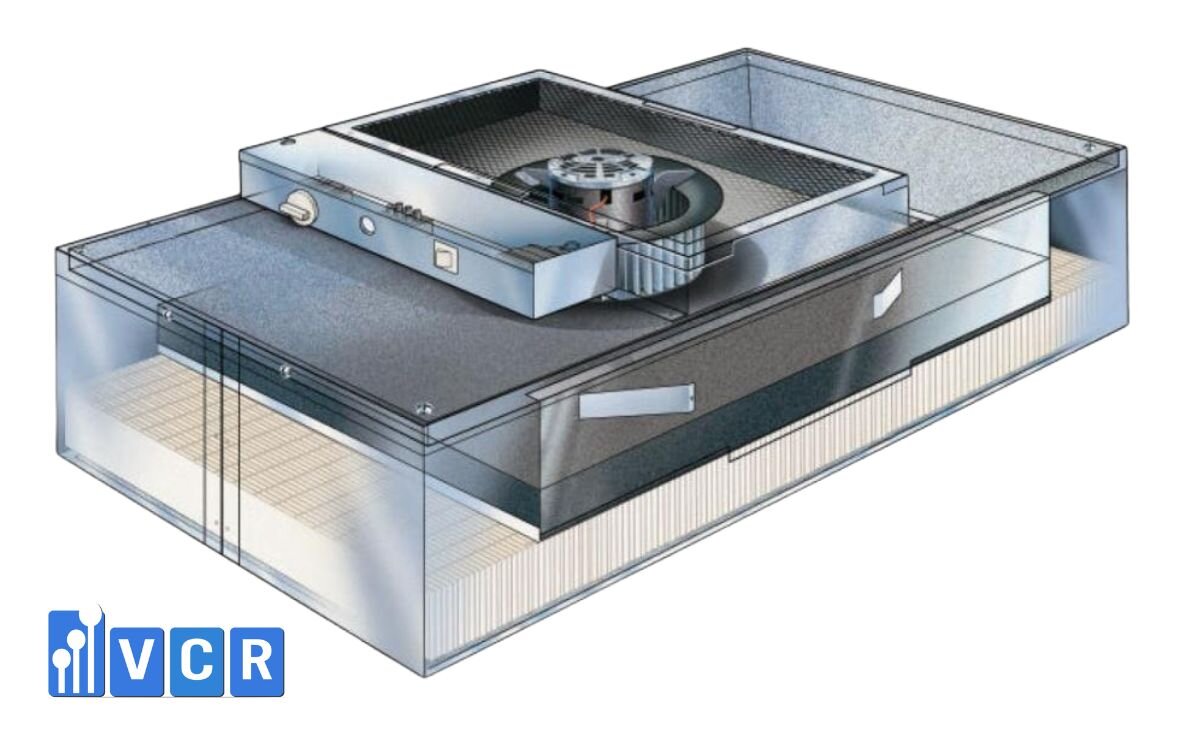
End-to-End Services - From Consultation to Validation
VCR not only supplies equipment but also supports clients throughout the entire project lifecycle:
- Cleanroom design consultation in compliance with GMP and ISO 14644.
- Supply of certified, high-quality equipment.
- Installation and commissioning according to validation protocols.
- Ongoing inspection and assessment to maintain GMP compliance.
With its “One-stop service” approach, VCR helps companies shorten project timelines, reduce costs, and gain confidence when undergoing GMP audits.
See more: The Evolution of the FFU: From Niche Tool to Cleanroom Champion
5. Comparison Between Standard FFU and GMP-Compliant FFU
Not all FFUs on the market meet GMP standards. There is a clear distinction between standard FFUs (for basic cleanrooms) and GMP-compliant FFUs (for pharmaceutical, food, and advanced electronics facilities).
|
Criteria |
Standard FFU |
GMP-Compliant FFU |
|
Filtration |
Basic HEPA filter only |
HEPA H14 or ULPA U15-U17, GMP-grade |
|
Standards |
Inconsistent, meets only low ISO levels |
Complies with EU/WHO GMP and ISO 14644 |
|
Applications |
Basic cleanrooms (ISO 7-9) |
GMP facilities, clean booths, weighing rooms, filling areas |
|
Reliability |
Moderate, difficult to validate |
High, fully certified, regularly validated |
|
Service life & operation |
3-5 years, difficult filter replacement |
5-7 years, modular, easy maintenance, energy-efficient |
Clearly, GMP-compliant FFUs excel in filtration efficiency, reliability, and compliance with international standards. They are essential for facilities seeking GMP certification.
See more: Can FFU (Fan Filter Unit) Be Connected to an Air Duct?
6. Frequently Asked Questions about GMP-Compliant FFUs
Are FFUs mandatory in GMP cleanrooms?
Yes. FFUs are especially required in areas classified ISO 5-7, such as weighing rooms, filling, and sterile packaging zones.

How is an FFU different from a HEPA Box?
FFU: equipped with an integrated fan, generates continuous laminar airflow.
HEPA Box: contains only a filter, relies on a central air system, does not generate airflow on its own.
What is the service life of an FFU?
On average 5-7 years, depending on operating conditions, usage, and maintenance frequency.
Does VCR provide customized FFUs?
Yes. VCR manufactures modular FFUs tailored to specific GMP projects, from small clean booths to large-scale cleanroom facilities.
7. Contact for Consultation & GMP-Grade FFU Quotation
Are you looking for a GMP-compliant Fan Filter Unit (FFU) solution for your pharmaceutical, food, electronics, or cosmetic factory? Let VCR Cleanroom Equipment be your trusted partner:
- Consultation on cleanroom design according to GMP and ISO 14644.
- Supply of internationally certified FFUs.
- Modular, customized solutions for specific projects.
- End-to-end services: design - installation - validation - maintenance.
Contact us today to receive a detailed quotation and free consultation from VCR experts.
Hotline: 090.123.9008
Email: [email protected]
Website: https://ffu.com.vn/
Diep VCR